- ARAB NEWS
- 15 Jun 2025

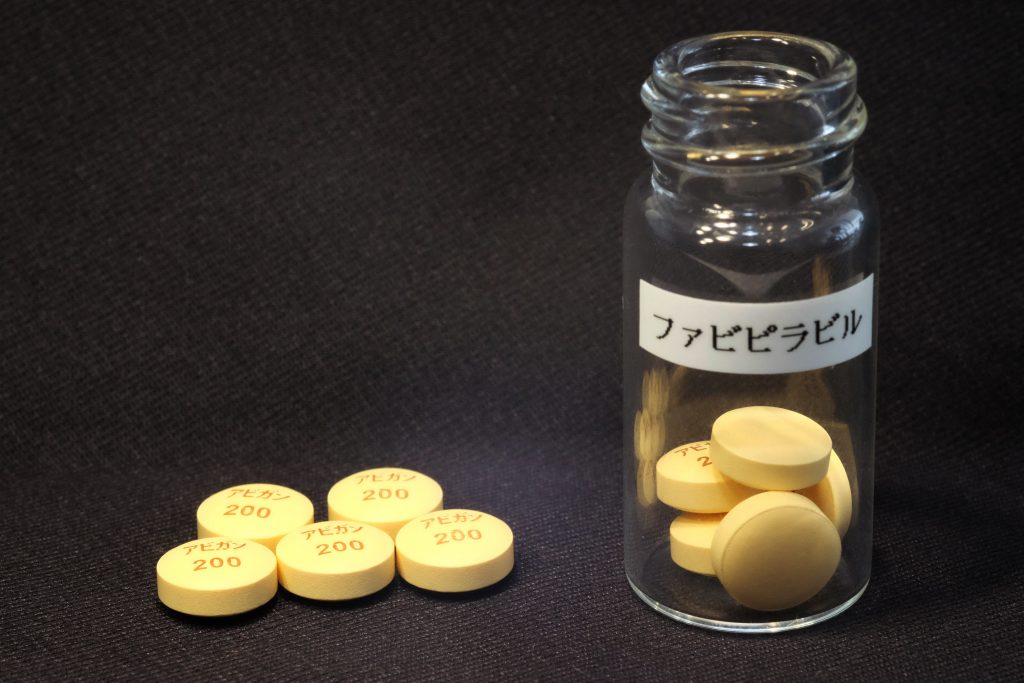
TOKYO: Fujifilm Toyama Chemical Co. said Wednesday that it has started a new Phase III clinical trial of its antiflu drug Avigan for use to treat COVID-19 patients in Japan.
The Fujifilm Corp. unit applied last October for approval of Avigan as a treatment for patients with the coronavirus disease, but Japan’s health ministry has found it difficult to confirm the drug’s efficacy until now.
Through the fresh final-stage clinical trial, Fujifilm Toyama Chemical plans to submit the additional data necessary for the drug to be approved.
The Phase III trial, planned to continue until late October, will cover as many as about 300 COVID-19 patients aged 50 or older, who have higher risks of developing severe symptoms within 72 hours of onset.
The company started a clinical trial on the use of Avigan for COVID-19 in March last year. It has since learned that the drug to some extent helps patients without severe symptoms to recover sooner.
In Japan, remdesivir and dexamethasone have already been approved as COVID-19 drugs. Avigan, whose generic name is favipiravir, is currently used for some patients with their consent in observation studies.
JIJI Press