- ARAB NEWS
- 01 Aug 2025

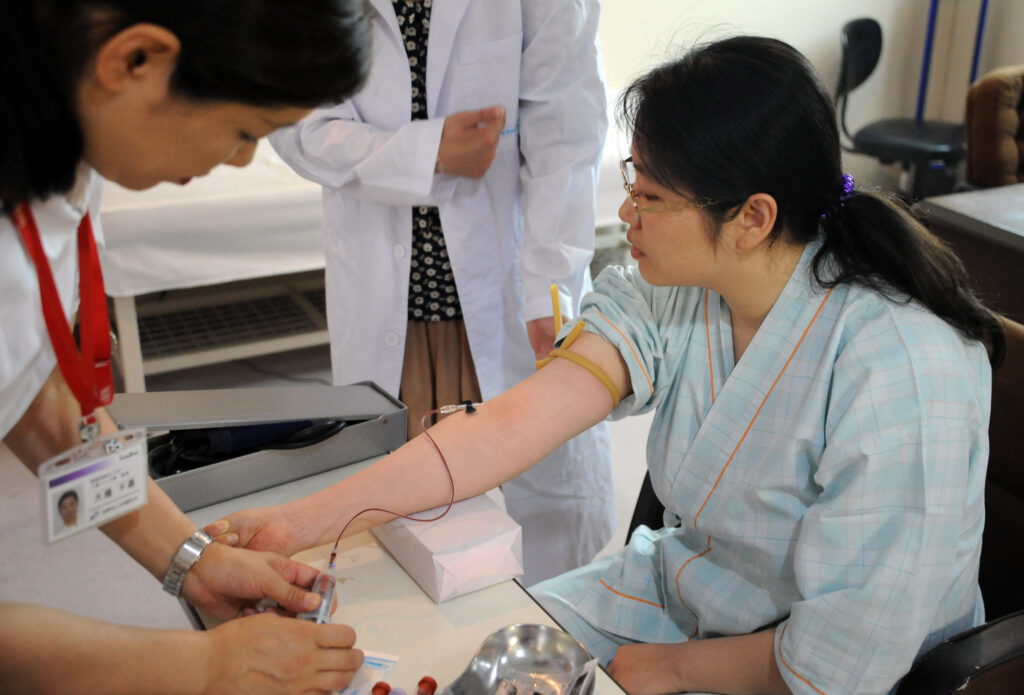
KASHIHARA, Nara Pref.: Nara Medical University will begin a clinical trial in spring 2025 of artificial red blood cells made from donated blood past its shelf life that is usually discarded.
A research team of the university in Nara Prefecture, western Japan, aims to put the artificial cells into practical use around 2030 amid growing interest in using such cells in the event of natural disasters and on remote islands.
The practical use of such cells, if realized, is expected to be the world’s first.
Artificial red blood cells are produced by extracting hemoglobin, which carries oxygen, from donated blood and wrapping it with an artificial lipid membrane, according to the team.
The artificial cells have a purplish color, unlike the common red color of blood, as they are made in a way that they do not oxidize until they are used.
Artificial red blood cells made with this method can be stored for up to about two years at room temperature and five years under refrigeration, while natural red blood cells can usually be stored for only up to about one month under refrigeration.
Furthermore, the original membrane that determines blood types has been removed, making blood typing unnecessary, a big advantage in emergency situations.
“When a blood transfusion is urgently needed, it takes some time before starting the transfusion because the patient’s blood type needs to be examined,” said Hiromi Sakai, professor at the university and a member of the team.
“With the artificial red blood cells, there is no need to worry about blood types, so the transfusion procedure can be performed quickly.”
In the clinical trial, 16 healthy adults will each receive up to 400 milliliters of artificial red blood cells. The team will confirm safety by administering the cells in various patterns with different infusion speeds and volumes.
When the cells are put into practical use, they will be used by the 800 milliliters, a widely used volume for medical treatment.
Artificial red blood cells “may be useful in the current situation in which blood donations are decreasing due to the country’s decreasing births and aging population,” Sakai said. “We will investigate thoroughly in this clinical trial and advance step by step toward practical use.”
JIJI Press