- ARAB NEWS
- 18 Jul 2025

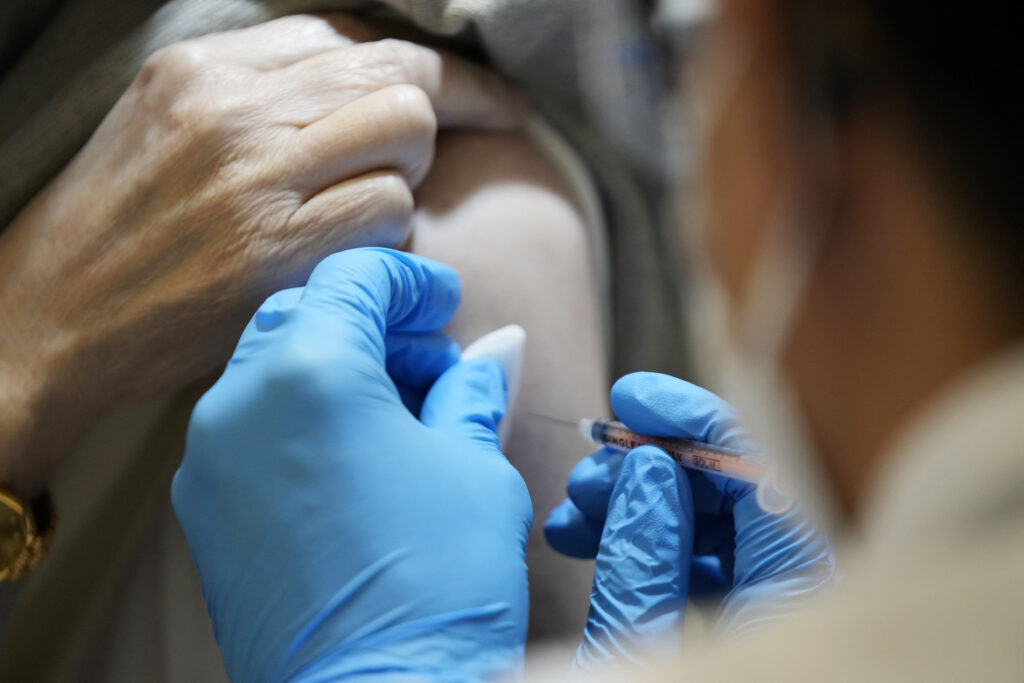
Tokyo: Japan’s health ministry Tuesday approved a COVID-19 vaccine developed by Japanese drugmaker Daiichi Sankyo Co.
The shot became the first approved homegrown vaccine against the XBB.1.5 subvariant of the omicron strain of the novel coronavirus.
The ministry has agreed to buy 1.4 million doses of the vaccine, which will be delivered nationwide from the week starting Monday and used for booster shots against COVID-19 for people aged 12 and older.
On Tuesday, the ministry also approved a novel coronavirus vaccine developed by a U.S. drug discovery startup. But the vaccine will not be supplied as it is against the novel coronavirus strain that spread in Wuhan, China, in the first phase of the pandemic.
Tokyo-based Meiji Seika Pharma Co., which sought the approval for the manufacturing and marketing of the vaccine in Japan, said that it is currently preparing for and conducting clinical trials of vaccines against coronavirus variants and aims to commercialize them in 2024.
JIJI Press