- ARAB NEWS
- 31 Jul 2025

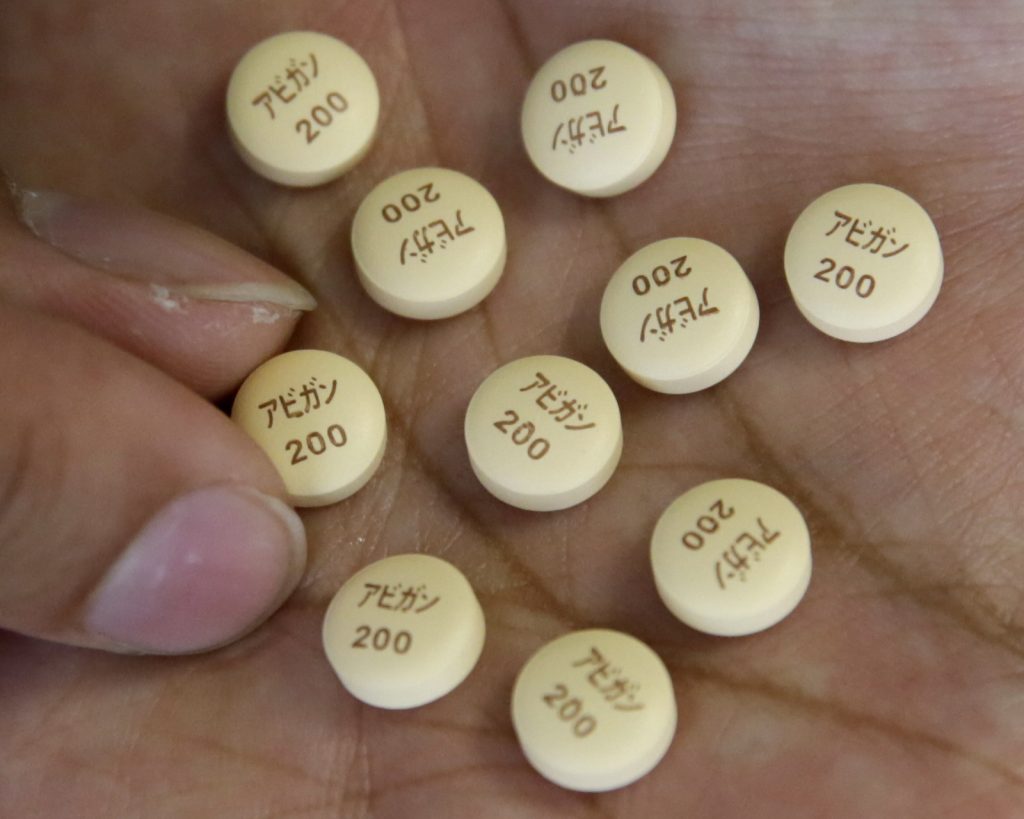
TOKYO: Japan is seeking to boost production of influenza drug Avigan, which is widely viewed as a potential treatment for the COVID-19 disease, caused by the novel coronavirus that is spreading across the country and elsewhere in the world.
The drug, developed by Tokyo-based Fujifilm Toyama Chemical Co., a Fujifilm Holdings Corp. subsidiary, is seen potentially curbing the reproduction of the coronavirus.
Expectations are running high for the drug, approved in Japan in 2014 as a cure for new-type influenza, for use as a remedy for the coronavirus, with a much shorter time taken for repurposing an existing drug than developing a new one.
Clinical trials are underway to examine the efficacy of the medicine, and it could be widely used for COVID-19 patients by year-end. The drug, however, cannot be administered to pregnant patients as its side effects include fetal malformation.
The Japanese government plans to earmark 13.9 billion yen for aiding increased production of Avigan under its fiscal 2020 supplementary budget, aiming to secure supplies enough for two million patients. The planned spending is part of its coronavirus-related emergency economic measures.
Fujifilm Toyama Chemical plans to increase production capacity, from making the drug for 40,000 patients a month to 300,000 patients, by September.
One obstacle has been securing the necessary materials. Fujifilm Toyama Chemical has depended heavily on imports from China for diethyl malonate, a key material for Avigan.
To help overcome the issue, midsize Japanese chemical maker Denka Co. will reopen its suspended facility in Niigata Prefecture, central Japan, to produce the compound from May, at the request of the government.
"It comes with difficulties, but our employees are burning with a sense of mission," Denka President Manabu Yamamoto said.
Domestic rivals Ube Industries Ltd. and Kaneka Corp. have also offered to supply materials for Avigan.
According to reports, a COVID-19 patient tested negative for the virus on the fourth day of being administered the drug at Tokyo Shinagawa Hospital.
Japanese Prime Minister Shinzo Abe said he wants to "spread the Japan-born miracle drug around the world," as many countries have asked for the supply of the drug.
Similar efforts to repurpose existing medications are being made.
A Japanese research institution has joined an international clinical trial to test the effects of remdesivir, an Ebola remedy by U.S. pharmaceutical firm Gilead Sciences Inc., to treat COVID-19 patients.
A U.S. medical journal reported that 36 of 53 severely ill patients saw their conditions improve as a result of being administered the drug.
But a Chinese clinical trial for the drug was reported to have ended in failure, leaving doubts about whether remdesivir can be used as a coronavirus remedy.
Clinical trials to test effectiveness for treating COVID-19 have also begun for the rheumatoid arthritis drugs Actemra, developed by Chugai Pharmaceutical Co. under the wing of Swiss pharmaceutical giant Roche, and Kevzara from French pharmaceutical giant Sanofi.
JIJI Press