- ARAB NEWS
- 15 Jun 2025

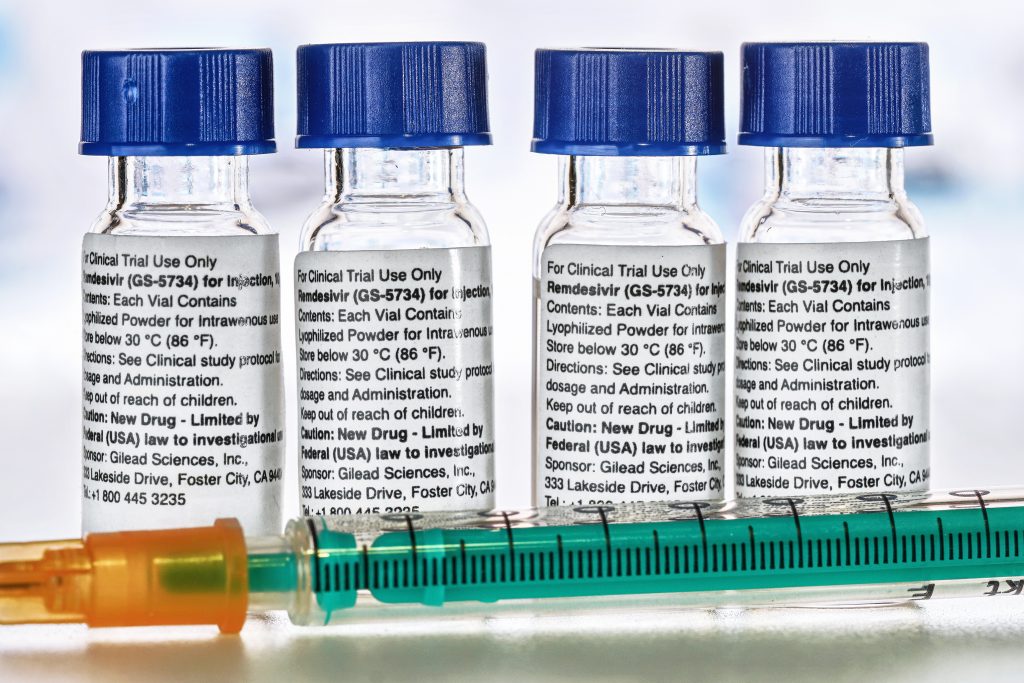
Gilead Sciences Inc's antiviral drug remdesivir may be approved in Japan for domestic COVID-19 patients when the health ministry's review board is held on Thursday, Japanese Health Minister Katsunobu Kato said.
Remdesivir was granted emergency use authorization last week by the U.S. Food and Drug Administration for COVID-19, the highly contagious lung disease caused by the new coronavirus, and Gilead filed for fast-track approval in Japan on Monday.
Kato said on a TV news programme on Tuesday if the review board gives its consent, he plans to approve it right away.
"We will approve (the drug) quickly if we receive endorsement from the Pharmaceutical Affairs and Food Sanitation Council,” Kato said
"Once imported, we would like to have it used by those who are suffering from serious conditions as soon as possible," Kato added.
Prime Minister Shinzo Abe on Monday extended a nationwide state of emergency to May 31, saying the new coronavirus infection rate had yet to drop enough to justify ending measures aimed at slowing the outbreak.
Even though Japan has not seen a huge outbreak compared with some global hotspots, there are more than 16,000 recorded cases, including 712 from the cruise ship previously
quarantined in Yokohama, and 579 deaths, according to public broadcaster NHK.
JIJI Press/Reuters