- ARAB NEWS
- 31 Jul 2025

Japanese pharmaceutical companies are scrambling to deliver their own drugs to treat the novel coronavirus amid intensifying international competition in a world gripped by the COVID-19 pandemic.
Many companies in and outside Japan are trying to repurpose antiviral drugs developed to treat other diseases. The leading example is Remdesivir, developed by Gilead Sciences Inc. of the United States as a possible treatment for Ebola. On Thursday, Japanese authorities approved it as the first COVID-19 drug in Japan.
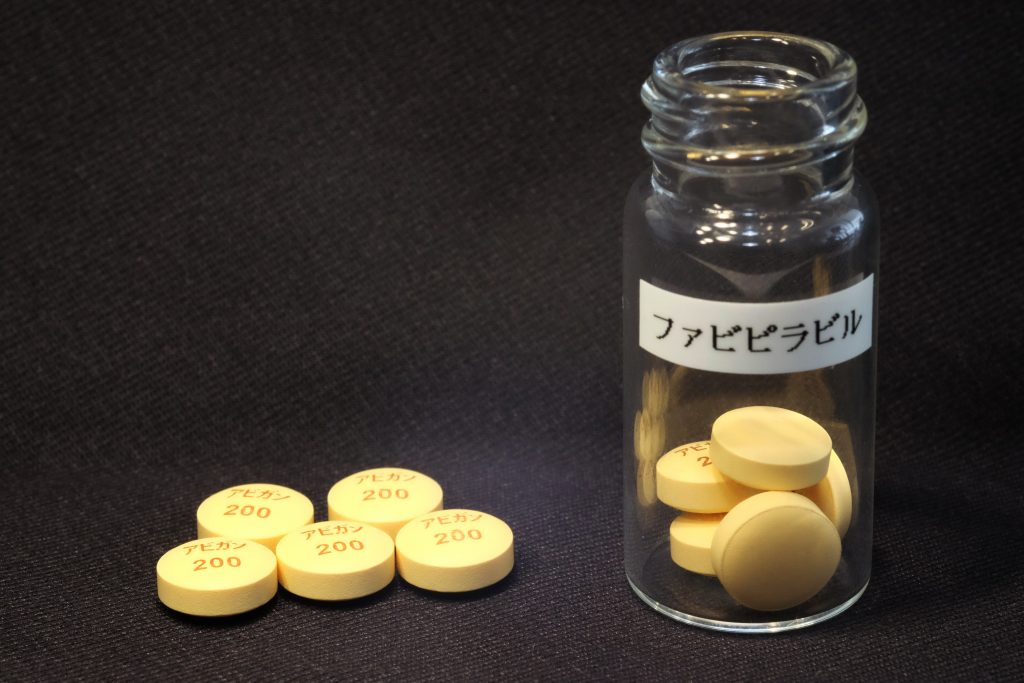
Fujifilm Toyama Chemical Co.'s influenza drug Avigan looks certain to follow Remdesivir. Prime Minister Shinzo Abe has said the government aims to approve the drug this month for treating coronavirus patients.
A clinical trial to verify the effectiveness and safety of Avigan in coronavirus treatment started in late March. The Fujifilm Holdings Corp. affiliate will work to establish a system to ensure stable supplies of the drug in Japan with support from the government.
Avigan, however, cannot be administered to pregnant women because of the risk of birth defects as a side effect.
Research is also under way on Alvesco, a steroid inhalant for asthma patients. The European-developed drug, produced for sale in Japan by Teijin Pharma Ltd., came under the spotlight after its effectiveness was cited in a report on the treatment of some patients from a cruise ship quarantined off Yokohama, Kanagawa Prefecture, earlier this year.
A clinical trial is expected to start this month for Chugai Pharmaceutical Co.'s Actemra, a rheumatoid arthritis drug.
The drug, which inhibits the activities of inflammation-causing substances, would be used for patients with severe symptoms. Chugai aims to apply for approval as a COVID-19 drug by year-end.
Meanwhile, Takeda Pharmaceutical Co. has started developing a new drug with antibodies in blood plasma from patients who recovered from COVID-19, using technologies of a major European drug maker Takeda acquired last year.
The company said it will collect blood plasma efficiently by hooking up with companies in Europe and the United States.
Shionogi & Co. aims at launching a clinical trial for a new COVID-19 drug by the end of next March. The company also plans to develop a vaccine against the disease and start a clinical trial by the end of this year.
Mitsubishi Tanabe Pharma Corp. will develop a vaccine at a Canadian unit.
Biopharmaceutical company AnGes Inc. hopes to start in July a clinical trial for a vaccine under development.
JIJI Press