- ARAB NEWS
- 01 Aug 2025

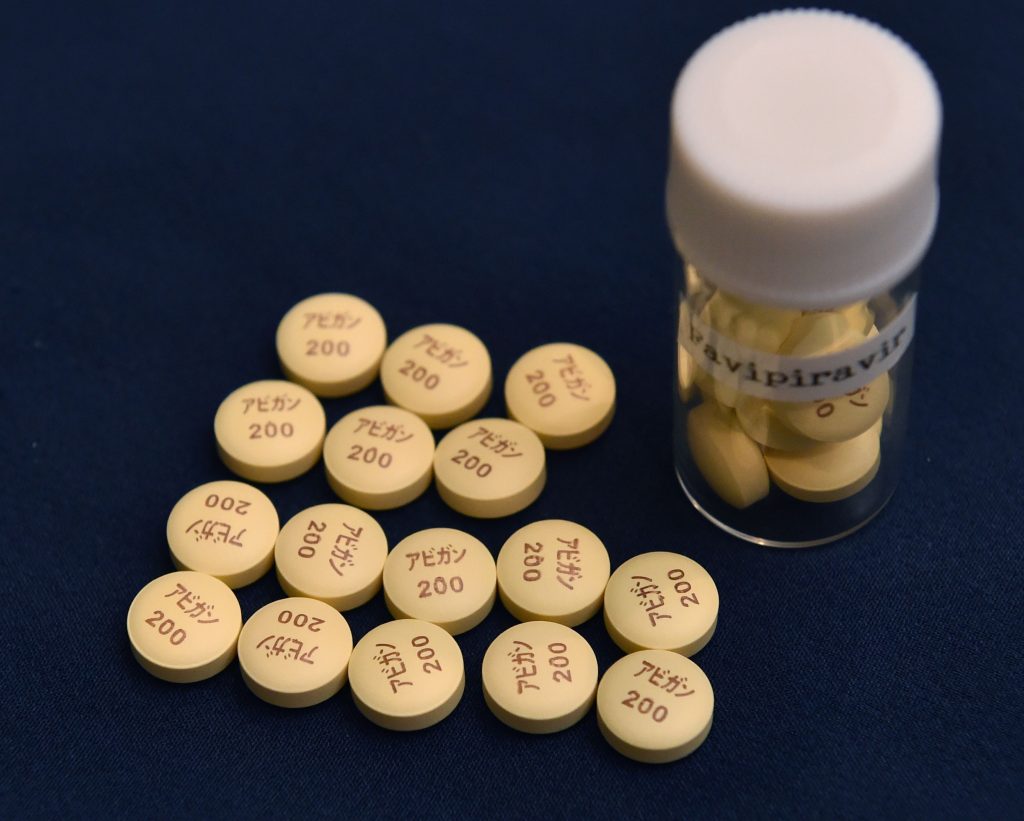
TOKYO: Japanese health minister Norihisa Tamura on Tuesday denied media reports that the government plans to approve the use of the influenza drug Avigan for patients with the novel coronavirus in November after three weeks of examination.
“There’s no way that the timing of approval is decided before an application is made,” Tamura told a press conference.
The health ministry will decide whether to approve a drug after checking its effectiveness and safety, Tamura said. “There would be no need to conduct an examination if the timing is determined beforehand,” he said.
In late September, Fujifilm Toyama Chemical Co., a Fujifilm Holdings Corp. subsidiary that developed Avigan, said that it will file for regulatory approval as early as October to use the drug as a COVID-19 treatment.
The company said that its clinical tests have found that Avigan is effective, to some extent, in treating patients with the respiratory disease caused by the new coronavirus.
Avigan has been approved in Japan as an antiflu drug, but concerns remain over its side effects such as birth defects.
JIJI Press