- ARAB NEWS
- 18 Jul 2025

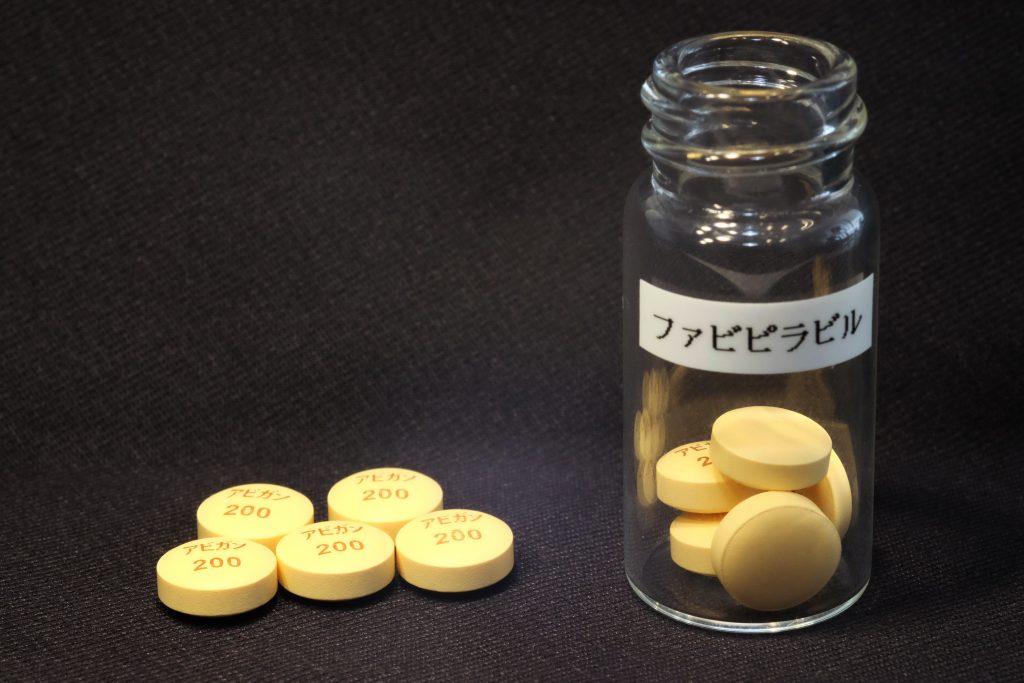
TOKYO: Japan has started to provide Avigan, a Japanese-developed antiflu drug that is expected to be effective against the COVID-19 infectious disease, for free to over 40 countries in the midst of the pandemic.
Through the initiative, Japan aims to accumulate clinical trial data on the drug and take the lead in the global race to find a cure for the coronavirus disease.
On Friday, Foreign Minister Toshimitsu Motegi said that Estonia has been the first country to be given the drug, adding that four more nations including Luxemburg will be provided with it in coming days.
As of Friday, Japan had agreed with 44 countries, also including Belgium and Iran, on the supply of Avigan. The number of recipient countries is seen reaching around 80.
"Avigan is drawing great attention from around the world," Motegi told a press conference.
"The world is eager to find an effective COVID-19 drug, which is needed for returning to daily life before the pandemic," a Japanese government source said. "By doing this, Japan can make great contribution."
Prime Minister Shinzo Abe and US President Donald Trump, during their telephone talks on Friday, confirmed their countries' cooperation to develop drugs for the disease.
Japan is using its official development assistance program to provide Avigan to other countries through the UN Office for Project Services. Each recipient country will be given the drug for 20 patients in principle or up to 100 patients.
Recipient countries have agreed to provide Avigan trial data to Japan, and not to hold Japan responsible for any problems from the use of the drug, which may have teratogenic effects.
In Japan, the process to approve Avigan as a treatment for COVID-19 is now in the final stages. Trial data from other countries can be utilized in the process, according to the health ministry.
JIJI Press