- ARAB NEWS
- 04 May 2024

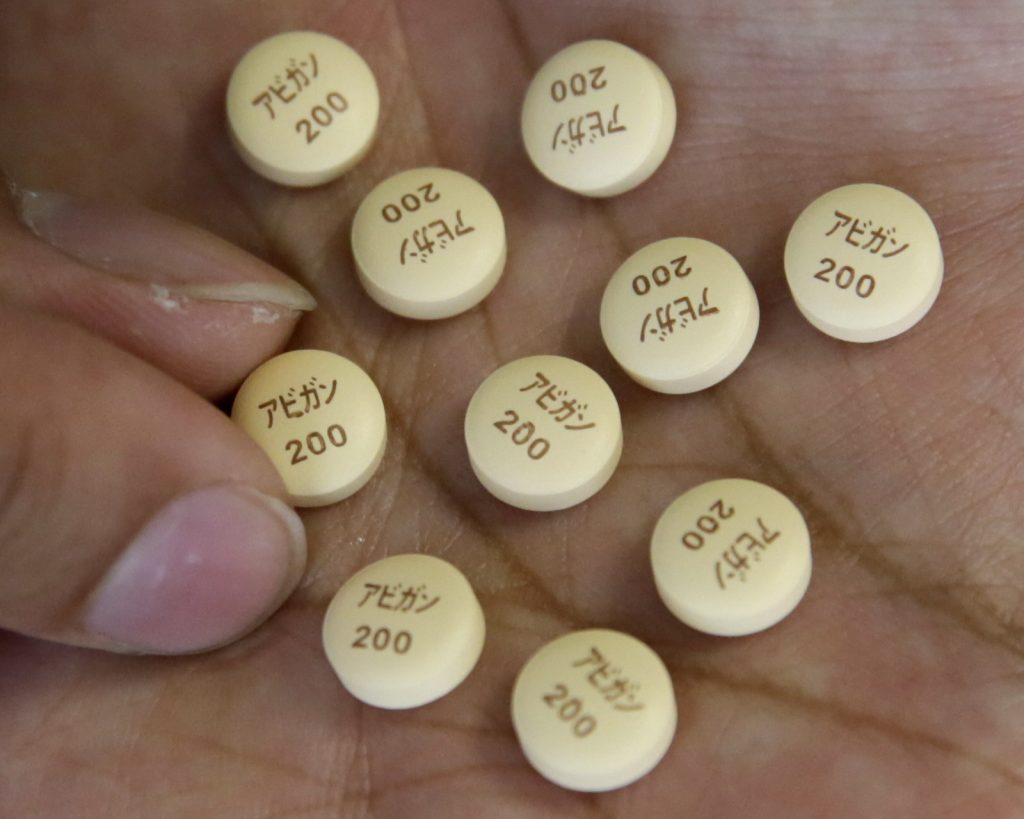
TOKYO: A Japanese health ministry panel has postponed a decision on whether to approve the use of the influenza drug Avigan as a treatment for COVID-19, citing the difficulty in determining the drug’s effectiveness with currently available data.
The Pharmaceutical Affairs and Food Sanitation Council will examine Avigan’s effectiveness again early next year, or later, if Fujifilm Toyama Chemical Co., a Fujifilm Holdings Corp. subsidiary that developed the drug, submits additional data from its ongoing clinical tests.
The panel decided to postpone its decision on Avigan at a working group session held on Monday.
Avigan, which has been approved in Japan as an antiflu drug, is believed to be effective in curbing the growth of the new coronavirus, which causes the COVID-19 respiratory disease.
In October, Fujifilm Toyama Chemical, based in Tokyo, applied for approval of Avian as a treatment for COVID-19, saying the drug was found through its clinical tests to be effective in shortening the time required for patients to produce negative results in polymerase chain reaction, or PCR, tests for the new coronavirus by 2.8 days.
If approved, Avigan would be the third COVID-19 treatment available in Japan, following remdesivir and dexamethasone.
According to the health ministry, in the clinical tests conducted by Fujifilm Toyama Chemical, doctors knew whether patients were given Avigan or a placebo.
The panel decided to continue its deliberations on Avigan after discussing at Monday’s meeting an allegation that the results of Fujifilm Toyama Chemical’s clinical tests are not strong enough to support the efficacy of the drug, compared to tests conducted with both doctors and patients not knowing whether the patients were given an actual drug or a placebo.
Avian has already been used for treatment of coronavirus patients who agreed to be part of an observation study. But it cannot be administered to pregnant women as some animal studies have pointed to risk of causing possible fetal malformation.
Former Japanese Prime Minister Shinzo Abe, who stepped down in September, initially sought to have Avigan approved as a domestically developed COVID-19 drug in May, but the government gave up on the plan as it took longer than expected to confirm the effectiveness of the drug in clinical studies.
“It’s very regrettable that the examination of the drug was extended,” Fujifilm Holdings and Fujifilm Toyama Chemical said in a comment released on Monday. “We’ll discuss our responses with the health ministry and others to realize an early approval.”
The companies said Avigan has been used for treatment of over 10,000 coronavirus patients under the observation study.
JIJI Press